Your Second Brain
for Regulatory Compliance
Automate 70-80% of regulatory authoring.
Reduce submission time from months to weeks.
Trusted by




















Across the drug lifecycle, regulatory teams manage expanding volumes of interdependent documents, where small inconsistencies introduce risk and cause significant downstream delays.
High-volume documentation to move from discovery to registration.
- IND packages (pre-cllinical, CMC, clinical plans).
- Protocols, amendments, IBs.
- CSRs, safety narratives, ISS/ISE.
- Early Module 2 and Module 3 summaries.
Complex submissions required to secure market authorization.
- Full CTD/eCTD dossiers (Modules 1–5).
- Country-specific variations and forms.
- HA queries, IRs, 483s, CRLs.
- Advisory committee briefing packages.
- eCTD publishing & technical validation.
Ongoing documentation to maintain global market access.
- Labeling updates and CMC variations.
- Renewals, supplements, market expansions.
- PSURs, PADERs, DSURs.
- Promotional and medical review packages.
- Registration maintenance & commitments.
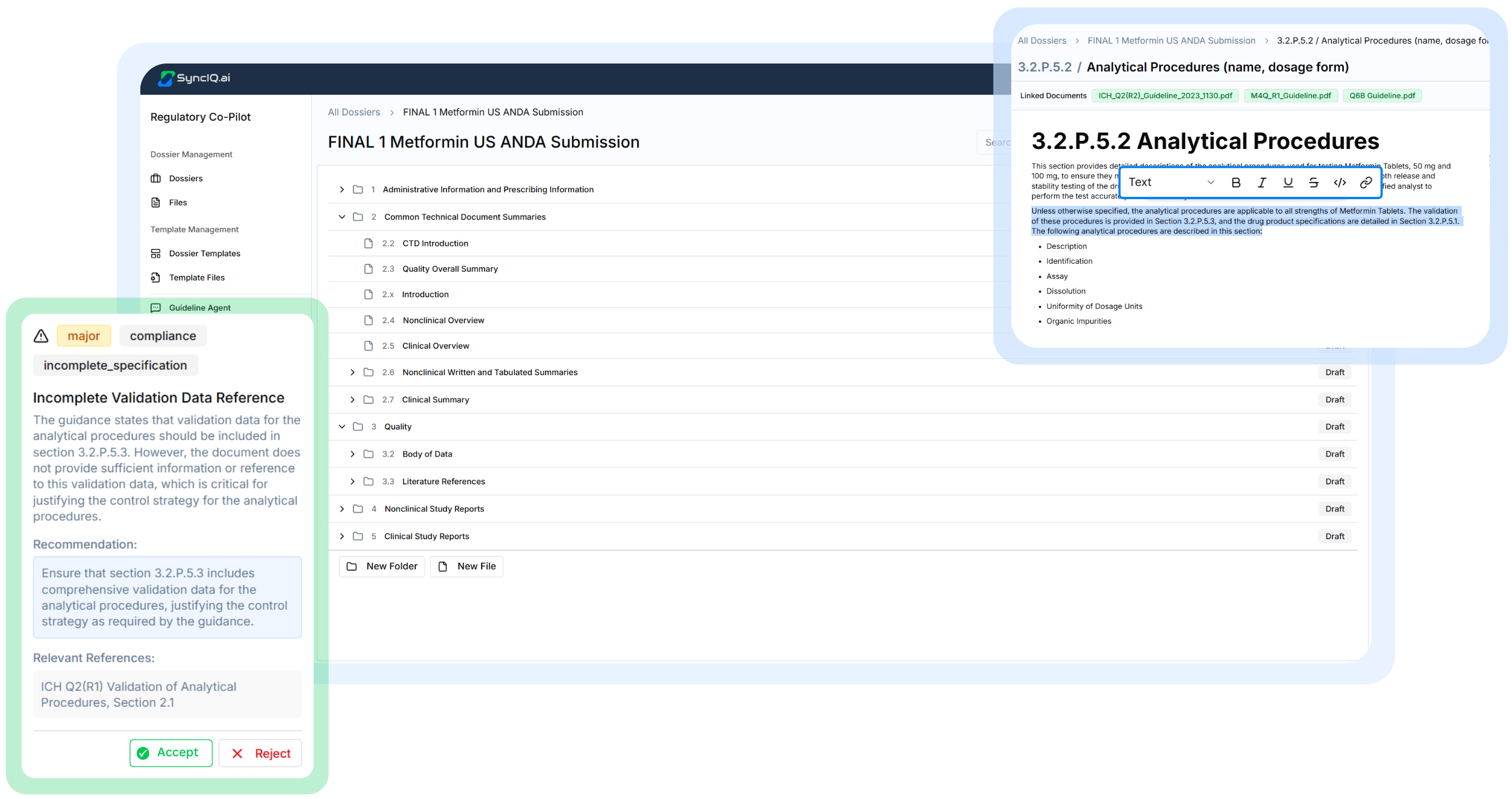
Built to handle the hardest parts of Regulatory Authoring
Each feature is designed around real submission workflows, helping teams draft, review, and maintain dossiers with speed, accuracy, and confidence.
Built to handle the hardest parts of Regulatory Authoring
Each feature is designed around real submission workflows, helping teams draft, review, and maintain dossiers with speed, accuracy, and confidence.
Proven impact for regulatory teams
From faster ANDA dossier authoring to fewer cross-document errors and quicker label update cycles, SyncIQ delivers real, auditable gains across the entire regulatory content lifecycle.
Modular Architecture for Limitless Automation
A flexible, layered design where projects, workflows, AI agents, and your data all connect seamlessly—empowering you to automate and scale any business challenge.
Your Command Center Layer
Unified Workspace: Create, launch, and manage all your business applications from one central hub.
Secure Permissions: Confidently manage access and permissions for every user and team.
Complete Audits: Maintains audit trails and version control for every app and workflow

Views & Dashboards Layer
Flexible data views for every role.
No-Code Builder: Instantly create custom tables, views, and dashboards—— no coding required.
Real-Time Visualization: Track, organize, and visualize your business data in real time.
Customizable Dashboards: Tailor dashboards to departments, teams, or individuals users for actionable insight.

Workflow Layer
Orchestrate data & process automation.
Visual workflow builder: Drag-and-drop task tuned agents to automate process steps, form fills, and table updates.
Direct Data Connection: Connect your workflows directly to tables and views for seamless data movement & control.
Human Oversight: Embed review & approval steps for human oversight where needed.
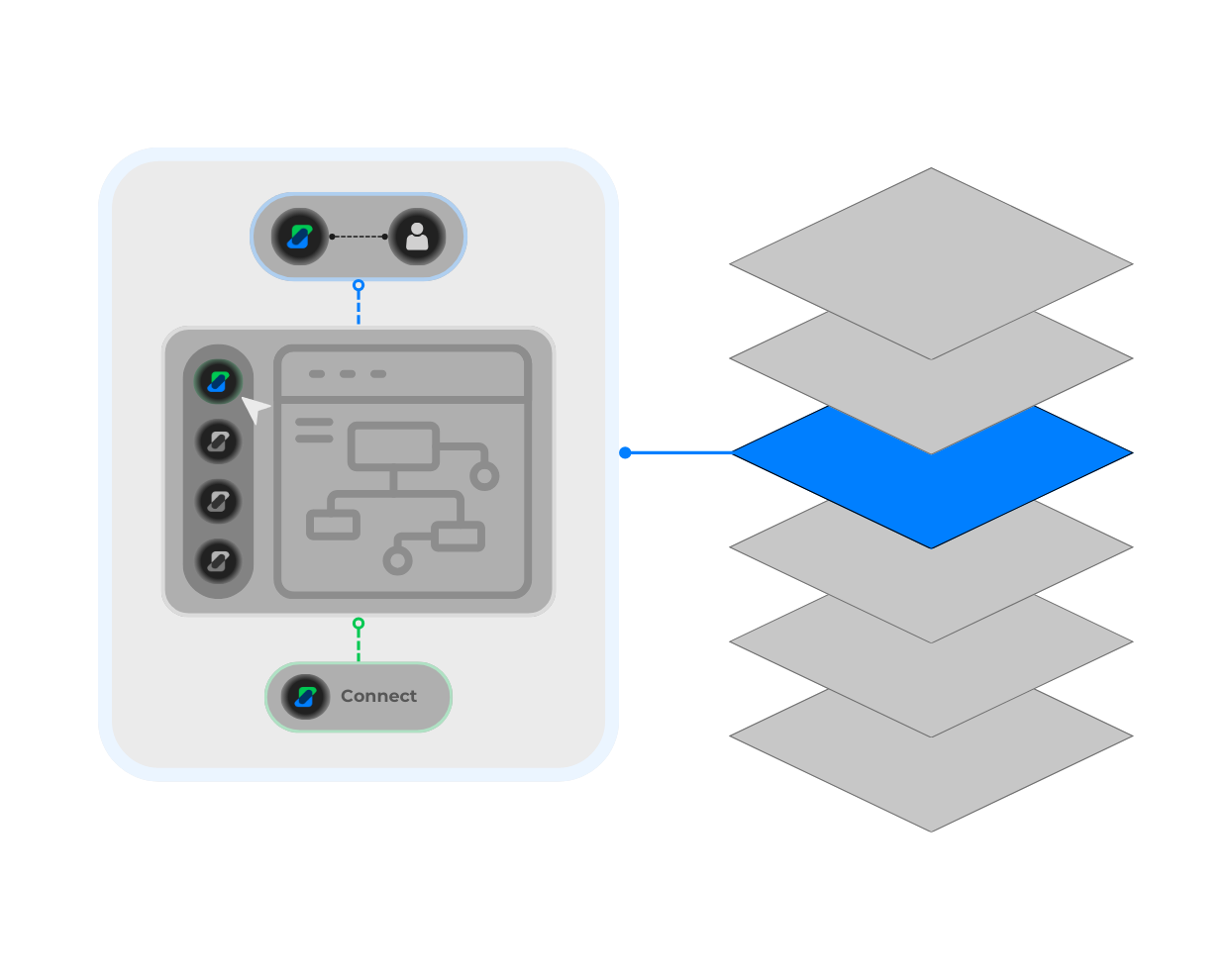
Agent Layer
Specialized AI Agents for every task.
Multi Step Agents: Orchestrate agents for complex, multi-step tasks.
Library of Agents: Access RAG Agents, document extraction agents, structured data agents, summarization agents, & custom agents.
Interaction: Enable chat-style agent-to-agent or agent-to-human conversations for better task coordination.

Designed to meet the standards of regulated, high-stakes submissions.
Fully traceable
All generated content maintains clear links to its original sources, enabling fast review, validation, and inspection-ready traceability.

Region-specific templates
Author content using predefined, region-aligned regulatory templates that guide structure, requirements, and formatting from the start.

Human in the loop
SyncIQ supports expert-led workflows where humans review, edit, and approve all content before submission or update.
Trusted by Regulatory, Clinical, and CMC teams worldwide
Set the pace for innovation in life sciences with SyncIQ
Learn how SyncIQ can help your organization file faster, scale smarter, and bring medical breakthroughs to patients months ahead of schedule.
